Unit 2 Chemistry
The Big Idea: The electron arrangement of atoms impacts their chemical nature.
|
Before you begin download the unit checklist:
|
| ||||||
Download PDF version of the Periodic Table
2.1 How and why do we study matter?
Handout: Key Terms
|
|
Chemistry |
|
|
Pure Substances vs. Mixtures |
|
|
Physical & Chemical Changes |
Handout: Elements, Compounds, and Mixtures
|
|
Lab Techniques & Safety |
Handout: Be Prepared; Be Safe
|
|
WHMISDownload the WHMIS handout and complete the 'Instant Practice - WHMIS' activity in your journal.
|
Handout: WHMIS 2015
Online WHMIS TrainingThe Canadian Worksite Safety Compliance Centre offers online WHIMS training for $29.95. This online training can be paid for by the school using your Family Account. This training is not a requirement of this high school science course, but it is a valuable employment skill. If you wish to take this online training please speak to your teacher.
|
Handout: Explore Safety Data Sheets
2.2 How does the periodic table organize the elements?
Handout: Elements on Brick World
|
|
Periodic TableDownload group names JPEG. You'll be expected to memorize these for the test.
|
|
|
Periodic Table Song |
|
|
How to Memorize the Periodic TableThis is just one of many ways to memorize. In this course you are expected to memorize the first 10 elements including: chemical name, chemical symbol, atomic number and location on the periodic table.
For more memorizing tips go to About Education |
|
|
Playlist: Periodic Videos |
|
|
Properties of Elements |
Online Activity: Interactive Periodic Table
Handout: Observing the Elements
Handout: Using the Periodic Table
Handout: Predict Properties
Handout: Other Contributors to the Periodic Table
Handout: What makes silicon so special?
Handout: Present an Element at ElementCon
Handout: Meet the elements
2.3 How can atomic theory explain patterns in the periodic table?
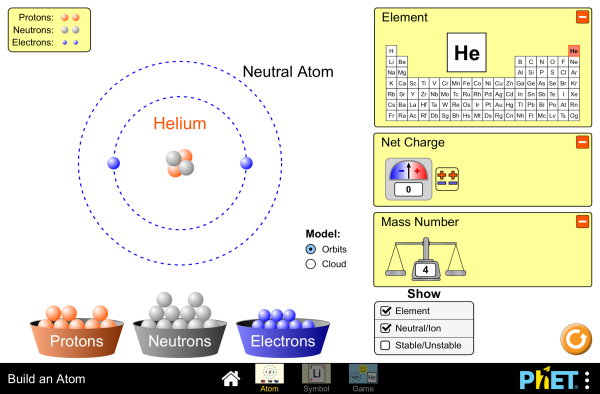
Handout: Model Bohr Atoms
Handout: Parts of an Atom
Handout: Bohr Diagrams
Handout: Valence Electrons and Group Numbers
|
|
Kinetic Molecular Theory |
|
|
Atomic Theory |
Online Activity: Build an Atomic Interactive
Handout: A Noble Gas is Hard to Find
2.4 How do elements combine to form compounds?
|
|
What are Covalent Compounds?Covalent compounds are also known as molecular compounds.
|
|
|
What are Ions & Ionic Compounds? |
Handout: Patterns in Ion Formation
Handout: Model a Compound
Handout: Comparing Ionic and Covalent Compounds
2.5 How do we name and write formulas for compounds?
|
|
Chemical NomenclatureDownload the following naming cheat sheet [source yourchemcoach.com]
|
|
|
Naming Ionic Compounds |
|
|
Writing Chemical FormulasPlay this Ionic Bonding Interactive
|
Handout: Names in Everyday Life
Handout: Naming Binary Ionic Compounds
Handout: Ion Ratios
Handout: Writing Names and Formulas of Binary Ionic Compounds Containing Multivalent Metals
Handout: Common Polyatomic Ions
Handout: Writing Names and Formulas of Compounds with a Polyatomic Ion
Handout: Writing Names and Formulas of Binary Covalent Compounds
Handout: How can you make a game out of names and formulas of ionic compounds?
Unit 2 Assessment
1. What are the effects of mining for metals and industrial minerals in B.C.?
2. Study for unit quiz
|
- memorize the first 10 elements of the periodic table. You will be required to know the following information about each of the 10 elements: chemical name and chemical symbol.
- be able to label the Group/Family names on a blank periodic table, including: alkali metals, alkali earth metals, transition metals, non-metals, halogens, noble gases, lanthanide metals and actinide metals - be able to name ionic compounds - be able to write ionic compounds from name - recognize if an compound is ionic or covalent |